SONODYNAMIC THERAPY IN RECURRENT HIGH-GRADE GLIOMA
About the Glioma
clinical trial
The Ivy Brain Tumor Center at Barrow Neurological Institute, a nonprofit translational research program, is conducting a Phase 0, first-in-human clinical trial of sonodynamic therapy (SDT) in ascending energy doses to assess safety and efficacy in patients with recurrent high-grade glioma.
In this study, sonodynamic therapy is the combination of the drug SONALA-001 (5-ALA, aminolevulinic acid HCl, or ALA) and a Magnetic Resonance-Guided Focused Ultrasound device called the Exablate 4000 Type-2 Device.
The goal of this study is to assess biological changes (tumor cell death) associated with the sonodynamic therapy.
Glioblastoma Trial Details
Recruiting
SONALA-001(ALA) and MR-Guided Focused Ultrasound device (MRgFUS)
High-Grade Glioma
30 Participants

You may be eligible if:
- You are 18 years or older
- You have had prior resection (surgical removal) of a histologically diagnosed high-grade glioma (III and IV)
- You have been treated with radiation and a chemotherapy drug called temozolomide
- Your doctor has told you that your tumor has recurred (come back) or has progressed (grown or changed)
How it works
The patient will be given aminolevulinic acid HCI (ALA) using an intravenous (IV) catheter 2, 4 or 6 days prior to a planned operation to remove their tumor.
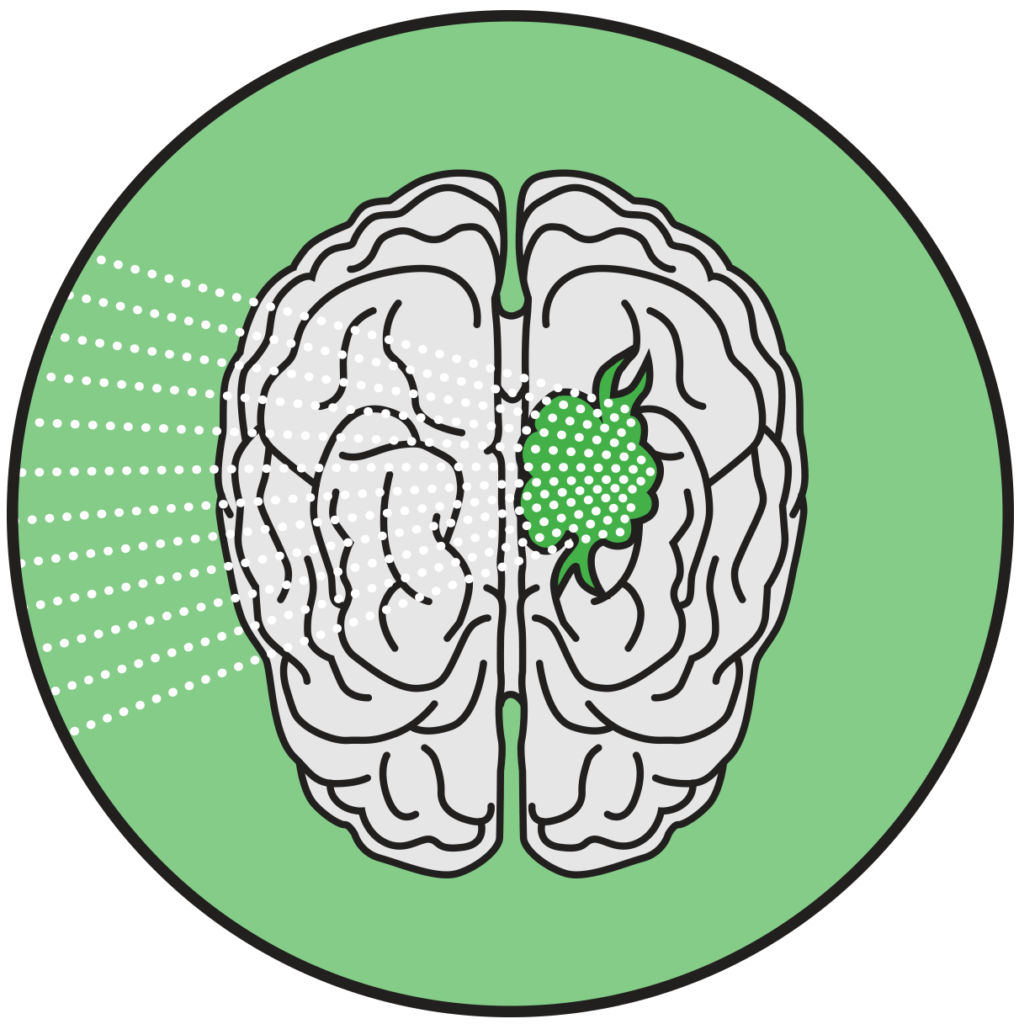
Approximately 6-7 hours after receiving ALA, they will undergo MR-guided focused ultrasound (MRgFUS) which will apply pulses of energy to activate the drug.

During surgery, tumor tissue samples will be collected so our team of experts can assess any biological changes (tumor cell death) associated with the sonodynamic therapy.
We will follow up with the patient for 30 days after surgery to assess safety of the therapy.
Am I Eligible?
Submit a free trial screening request today to learn if you may qualify for any of our studies.


