ONC201 IN NEWLY DIAGNOSED H3 K27M-MUTANT DIFFUSE GLIOMA
ABOUT THE H3 K27M-MUTANT DIFFUSE GLIOMA CLINICAL TRIAL
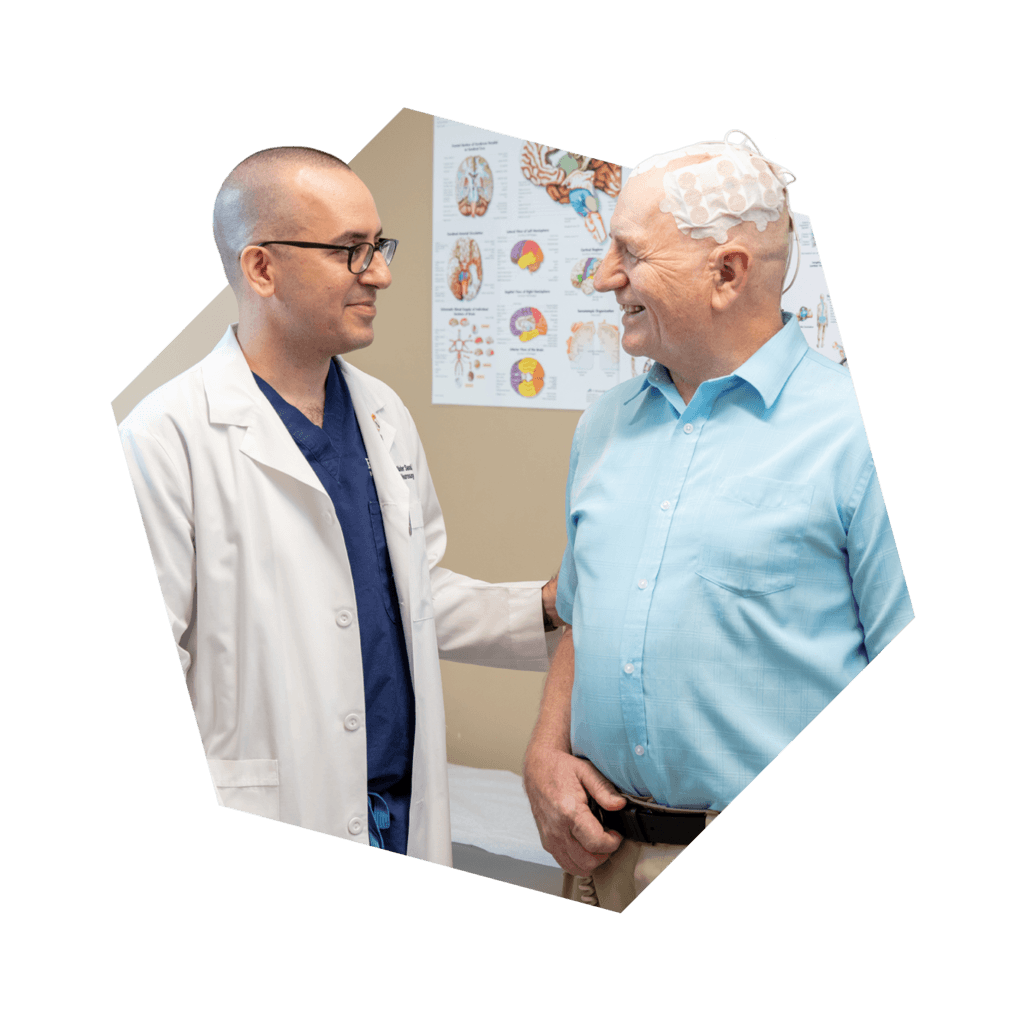
The Ivy Brain Tumor Center at Barrow Neurological Institute, a nonprofit translational research program, is conducting a Phase 3 study to evaluate ONC201 in patients with newly diagnosed H3 K27M-mutant diffuse glioma.
ONC201, a new, investigational drug being developed for glioma treatment, aims to treat tumor cells without affecting the body’s normal cells. ONC201 is a first-in-class drug, which means it is a new drug having unique action, and it is not yet approved by the U.S. Food and Drug Administration (FDA) for treating glioma. It can only be used in a study like this one.
The primary objective of this study is to assess the effectiveness of ONC201 in treating glioma patients following radiation therapy. The study aims to evaluate the safety and tolerability of ONC201 compared to a placebo (a capsule that looks like ONC201 but does not contain any active drug) and investigate if ONC201 can improve the quality of life and brain functioning in people with glioma.
Diffuse Glioma Trial Details
Recruiting
ONC201
Newly Diagnosed H3 K27M-mutant Diffuse Midline Glioma
450 Participants
Chimerix, Inc.
You may be eligible if:
- You are 18 years or older.
- You have a newly diagnosed H3 K27M-mutant diffuse midline glioma and it has not recurred following standard treatment.
- You have completed or plan to complete standard frontline radiotherapy within 12 weeks from initial diagnosis.
View all eligibility criteria by visiting ClinicalTrials.gov or call 602-406-8605 to speak with a patient navigator.
HOW IT WORKS
A patient with newly diagnosed H3 K27M-mutant diffuse glioma receives approximately six weeks of radiation therapy as part of standard of care.
Two to six weeks following radiation, the patient is enrolled in the Phase 3 study using ONC201, and a computer randomly assigns them to one of three study treatment groups.

This is a placebo-controlled study, meaning some subjects get ONC201; the others get a mix of ONC201 and placebo. The study drug will be administered orally two consecutive days per week and will continue until the participant meets criteria for discontinuation.

If the participant or their study doctor decides to stop the study drug, the participant will return to the clinic for a safety follow-up visit. They will then be contacted approximately every two to three months to ask how they are doing. The participant will also be asked to have an MRI every eight weeks until they start a new anticancer treatment.
Am I Eligible?
Submit a no-cost trial screening request today to learn if you may qualify for a clinical trial or talk to an Ivy Navigator by calling 602-406-8605.