AZD1390 FOR GRADE 4 GLIOMA
About The Grade 4 Glioma
clinical trial
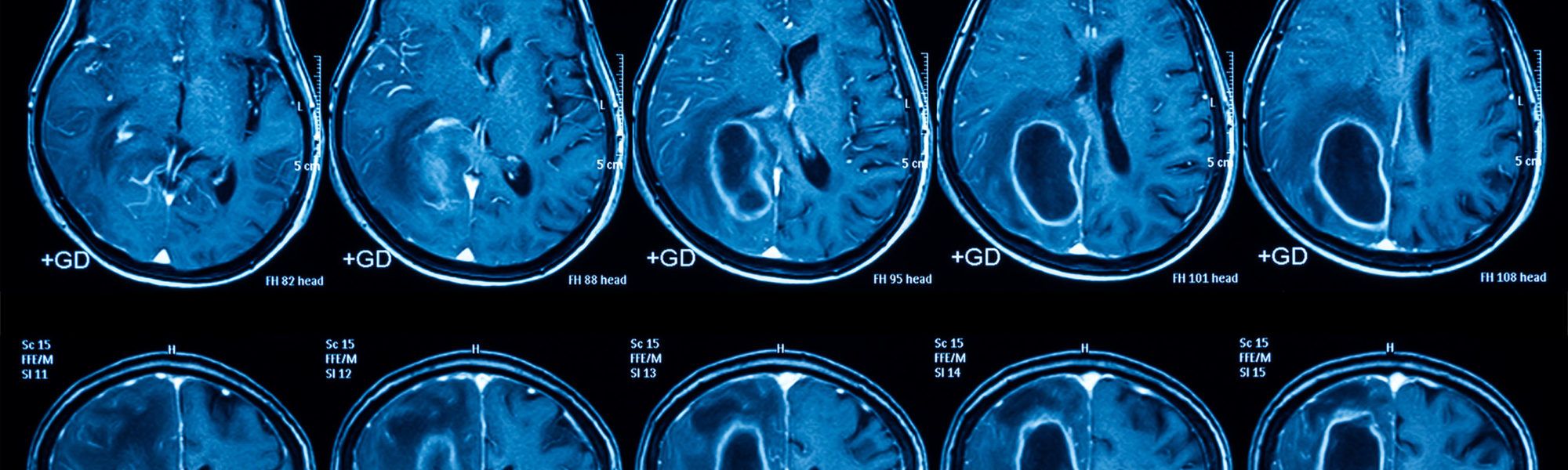
The Ivy Brain Tumor Center at Barrow Neurological Institute, a nonprofit translational research program, is conducting a Phase 0/1b clinical trial to evaluate AZD1390 in patients with recurrent and newly diagnosed WHO grade 4 glioma.
The goal of Phase 0 is to confirm that AZD1390 is capable of crossing the blood-brain barrier. Patients with positive results may advance to the 1b expansion phase which combines therapeutic dosing of AZD1390 with standard-of-care fractionated radiotherapy.
The expansion phase will examine progression-free survival and monitor safety and tolerability of AZD1390 in combination with radiation.
- AZD1390 is a highly potent brain penetrant ATM (Ataxia telangiectasia mutant) kinase inhibitor that blocks the repair of DNA breaks caused by radiation treatment.
- AZD1390 is in early clinical development for use as a radiosensitizer in central nervous system malignancies.
- Radiosensitizers make tumor cells more vulnerable to radiation therapy.
Grade 4 Glioma Trial Details
Recruiting
AZD1390
Recurrent and Newly Diagnosed WHO Grade 4 Glioma
47 Participants
You may be eligible if:
- You are 18 years or older.
- You have a suspected newly diagnosed WHO grade 4 glioma and plain to follow the standard treatment regimen.
-OR-
- You are 18 years or older
- You have had prior resection (surgical removal) of a diagnosed WHO grade 4 glioma and followed standard therapy, including temozolomide and fractionated radiotherapy.
- Your doctor has told you that your tumor has recurred (come back) or has progressed (grown or changed) and you require resection with radiation therapy as part of your post-surgical treatment plan.
View all eligibility criteria by visiting ClinicalTrials.gov or call 602-406-8605 to speak with a patient navigator.
Phase 0 Clinical Trial: How it works

Once enrolled, a patient receives a short exposure to the experimental therapy days before a planned operation to remove their tumor.

This exposure is enough that when we remove the tumor, our team of experts can answer an important question: Did the treatment penetrate the tumor?

If the drug penetrates the tumor at sufficient levels, the patient may move forward with receiving the treatment in combination with fractionated radiotherapy in the therapeutic expansion phase.

Alternatively, if the treatment has no effect on the tumor, the patient can enroll in another clinical trial without losing time or receiving ineffective treatment.
Am I Eligible?
Submit a free trial screening request today to learn if you may qualify for a clinical trial or talk to an Ivy Navigator by calling 602-406-8605.


