
Understanding Glioblastoma: Symptoms, Treatment Options, and Prognosis
Glioblastoma (GBM)
Glioblastoma is one of the most complex and treatment-resistant cancers, making it particularly daunting for patients and their families. Often, the first time someone encounters the word “glioblastoma” is when a diagnosis is made, bringing with it overwhelming feelings of fear, anxiety and uncertainty. At the Ivy Brain Tumor Center, we are committed to empowering patients with the knowledge and resources they need to navigate this challenging diagnosis. Explore our comprehensive guide to glioblastoma for more information.
Understanding Glioblastoma
What is glioblastoma?
Glioblastoma is a malignant (cancerous) primary brain tumor, meaning it originates in the brain as opposed to tumors that have metastasized from cancerous cells in another part of the body. These glioblastoma tumors arise from abnormal glial cells in the brain.

What are the different types of glioblastoma?
Glioblastoma, also known as glioblastoma multiforme (GBM), is classified by the World Health Organization (WHO) as a grade 4 tumor, the most aggressive form. The WHO recently updated its classification of central nervous system tumors, further distinguishing glioblastoma from astrocytoma. Under this new system, tumors formerly identified as glioblastoma, IDH-mutant are now classified as astrocytoma, IDH-mutant, grade 4.
Today, glioblastomas are specifically defined as astrocytoma, IDH-wild type tumors, as they do not harbor an IDH mutation. They must exhibit at least one of the following characteristics: microvascular proliferation, necrosis, EGFR amplification, TERT promoter mutation, or a combined gain of chromosome 7 and loss of chromosome 10 copy number changes.
How common is glioblastoma?
More than 14,000 glioblastoma brain tumors are diagnosed in the United States each year. It is most common in older adults, ages 65-74, but can occur at any age and is more common in men than women.
Glioblastoma is the most common malignant tumor of the brain and central nervous system (CNS), accounting for 47.7 percent of all cases. Its incidence is 3.21 per 100,000 people.
glioblastoma brain tumors
are diagnosed in the U.S per year
Is glioblastoma genetically hereditary?
Most cases of glioblastoma develop randomly and have no genetic basis. In rare cases, glioblastoma can be part of familial genetic syndromes, such as Turcot syndrome, neurofibromatosis type 1, and Li-Fraumnei syndrome. Mutations of specific genes may increase the risk of glioblastoma. There is ongoing research to find additional hereditary causes.
Glioblastoma Diagnosis
What causes glioblastoma?
The exact cause of glioblastoma is still largely unknown, but several factors may contribute to its development.
- Genetic Mutations: Abnormalities in genes that regulate cell growth and division can lead to the development of glioblastoma. Common mutations involve genes such as EGFR and TERT.
- Environmental Factors: While research is ongoing, certain environmental exposures, such as ionizing radiation (particularly from previous cancer treatments), have been linked to an increased risk of developing glioblastoma. However, there is no evidence that routine exposure to diagnostic radiation from imaging techniques, such as X-rays and CT scans, is associated with an increased risk.
- Age: Glioblastoma is more common in older adults, with the highest incidence occurring between the ages of 45 and 70.
- Gender: Men are more likely than women to develop glioblastoma.
- Family History: A family history of brain tumors or genetic syndromes (like Li-Fraumeni syndrome) may increase the risk.
While these factors may be associated with an increased risk, most cases of glioblastoma occur without any known risk factors, highlighting the complexity of its causes. Ongoing research aims to better understand the underlying mechanisms and potential risk factors for glioblastoma.
How is glioblastoma diagnosed?
The pathologist’s review of the tumor tissue confirms the diagnosis of glioblastoma. The tumor’s molecular profile determines the exact diagnosis. Advances in understanding the molecular genetics of tumors have resulted in more accurate diagnosis and treatment.
Glioblastoma Prognosis and Survival
What is the average survival rate for glioblastoma patients?
Survival for those with glioblastoma is poor, with approximately 40 percent survival in the first year after the diagnosis and 17 percent in the second year. Less than 7 percent survive more than five years, and the typical length of median survival for patients is only 8-16 months. It’s estimated that about 10,000 Americans will succumb to glioblastoma each year. These statistics haven’t improved in decades.
What factors influence the prognosis of glioblastoma?
Various factors can impact the prognosis of glioblastoma, and. understanding these can help inform treatment decisions and offer patients and their families clearer expectations regarding outcomes. Key factors include:
Age
Younger patients generally have a better prognosis compared to older individuals.
Karnofsky Performance Status (KPS)
This scale measures a patient’s ability to perform daily activities. A higher KPS score typically indicates a better prognosis.
Extent of Tumor Resection
The amount of tumor that can be surgically removed safely significantly impacts prognosis. More extensive resection is associated with improved survival rates.
Tumor Genetics
Genetic mutations in the tumor, such as those in the MGMT promoter, can influence treatment response and survival
Molecular Features
The presence of specific molecular markers, such as MGMT methylation, can affect the tumor’s response to chemotherapy and impact prognosis.
Treatment Response
How well the tumor responds to initial treatments, including surgery, chemotherapy, and radiation therapy, can influence overall survival.
Recurrence Patterns
The location and nature of tumor recurrence also play a role in prognosis. Recurrences in specific areas may be more challenging to treat effectively.
Overall Health
The patient’s general health and comorbid conditions can affect treatment options and outcomes.
How does MGMT methylation affect the prognosis of glioblastoma?
MGMT methylation plays a significant role in the prognosis of glioblastoma patients. MGMT is a DNA repair enzyme that fixes damage caused by chemotherapy agents like temozolomide (TMZ). When the MGMT promoter is methylated, the gene is silenced, which impairs the tumor’s ability to repair DNA damage from chemotherapy, making the tumor more responsive to TMZ. This methylation is present in approximately 40 percent of GBM patients, leaving around 60 percent with unmethylated MGMT promoters. Although patients with unmethylated MGMT generally have a reduced response to TMZ, it remains a standard treatment combined with radiation therapy and as monotherapy after the completion of radiation. Clinical trials may offer alternative therapies or enhance treatment outcomes for unmethylated MGMT patients.
Methylated MGMT
DNA repair enzyme is unable to repair the damage by chemotherapy and may respond better to temozolomide.
Unmethylated MGMT
DNA repair enzyme is able to repair the damage by chemotherapy and generally has a reduced response to temozolomide.
Glioblastoma Symptoms
What are the common symptoms of glioblastoma?
Glioblastoma symptoms can vary depending on the location and size of the brain tumor. Symptoms can present suddenly as the tumors grow rapidly and put pressure on the brain. The typical symptoms of glioblastoma include:
General symptoms of brain tumors include:
- New onset or change in pattern of headaches
- Headaches that gradually become more frequent and more severe
- New onset of seizures
- Gradual loss of sensation or movement in an arm or a leg
- Difficulty with balance
- Difficulty speaking
- Personality or behavior changes
- Confusion
- Unexplained nausea or vomiting
- Blurred vision, double vision, or loss of peripheral vision
- Hearing problems
Contact your physician if you are experiencing any of these symptoms.
How quickly does glioblastoma grow?
Glioblastoma symptoms can increase in severity as the tumor grows, and you may experience additional symptoms as the tumor affects more parts of the brain. Glioblastoma typically grows rapidly, often doubling in size within a few weeks. The exact growth rate can vary depending on factors such as the tumor’s location, the patient’s overall health, and treatment, but in general, glioblastoma is known for its ability to invade surrounding brain tissue quickly.
Without treatment, glioblastoma progresses very rapidly, leading to significant symptoms in a matter of weeks to a few months.
Glioblastoma Treatment Options
Standard of Care: What are the standard treatment options for glioblastoma?
Each patient’s glioblastoma brain tumor is unique and requires a personalized treatment plan. Typical treatment for glioblastoma consists of surgery followed by radiation and chemotherapy.
The Role of Surgery in Glioblastoma Treatment
The goal of surgery is to remove as much of the tumor as possible without disturbing nearby healthy and delicate brain tissue. Glioblastoma poses a challenge to neurosurgeons because the tumor’s microscopic cells are impossible to remove completely. They invade surrounding healthy tissue and allow the tumor to grow back or recur. Some patients experience deficits after brain surgery, such as a loss of mobility or cognitive function. Research has demonstrated that outcomes and preservation of neurologic function are better at centers with neurosurgeons specializing in brain tumors. Functional brain mapping and awake brain surgery can help surgeons remove more tumor cells safely. Neurorehabilitation after surgery can help patients recover from deficits. Learn the top four tips for faster healing after brain surgery.
Radiation Therapy for Glioblastoma: What To Expect
Most glioblastoma patients receive a standard treatment regimen of radiation and chemotherapy. Radiation therapy uses ionizing radiation directed at tumor cells, causing DNA damage and preventing malignant cells from multiplying. A unique radiation plan is created for each patient, ensuring safety and accuracy to preserve healthy brain tissue while effectively treating the tumor. Side effects from radiation therapy can include hair loss, skin irritation, fatigue, nausea, and a drop in blood counts.

Chemotherapy: How It Works for Glioblastoma
The standard chemotherapy drug for glioblastoma patients is temozolomide, also known as Temodar, which can be administered orally or intravenously. This medication has been used for decades to treat glioblastoma and has been shown in clinical trials to change the survival in those with unmethylated MGMT promoters minimally. Notably, the FDA has not approved a new life-prolonging drug for glioblastoma in nearly 20 years. While chemotherapy can be effective, it often causes side effects such as nausea, vomiting, and a drop in blood counts.
Despite these treatments and therapies, there is currently no cure for glioblastoma.
Neuro-oncology for brain tumors
Tumor Treating Fields
Tumor Treating Fields is a noninvasive technique delivered through Optune®, an FDA-approved device that a physician may prescribe as part of a treatment plan for appropriate patients after completion of radiation therapy. Worn on the head throughout the day, this device may help extend survival for some patients, according to recent studies. It is believed to work by disrupting cancer cell division with alternating electric fields applied through adhesive pads on the scalp.
Glioblastoma Recurrence: What if the tumor comes back?
The standard of care for glioblastoma is not curative, and patients will experience a recurrence of their brain tumor. Even after maximum surgical resection, microscopic glioblastoma cells remain and grow over time. To monitor for tumor regrowth, patients are scheduled for follow-up visits and routine MRI scans. When a recurrence is detected, patients may undergo additional surgery to remove as much of the new tumor as possible. The neuro-oncologist will reevaluate the situation and provide treatment recommendations tailored to the patient’s needs. Whether a patient is newly diagnosed or facing a recurrence, new treatment options, including clinical trials, are available to offer hope.
Emerging Treatments and Clinical Trials for Glioblastoma: How are treatment plans for glioblastoma personalized?
Clinical Trials
Brain tumor clinical trials provide glioblastoma patients with innovative treatment options when standard care proves ineffective and may be recommended as a first-line option in some instances.
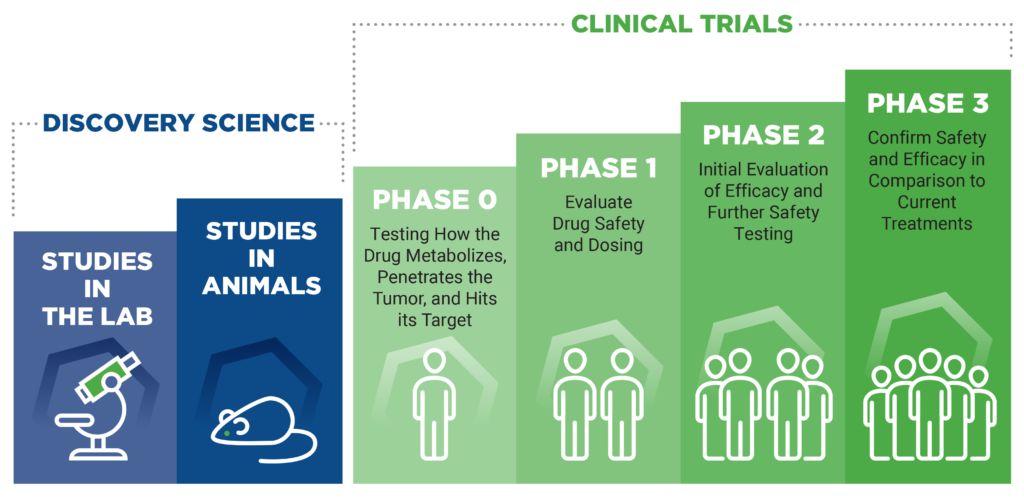
Clinical trials test new drugs, interventions and diagnostic tools that the FDA has yet to approve and offer patients access to cutting-edge therapies that are not widely available. Through clinical trials, researchers advance their understanding of brain tumors and inspire the development of novel therapies that can significantly impact patient outcomes.
Glioblastoma is the primary focus of the Ivy Brain Tumor Center and represents a significant portion of our clinical trials portfolio.
If you are interested in whether you or a loved one may qualify, please submit a free trial screening request or call 602-406-8605. An Ivy Navigator will complete the initial assessment within two business days.
Targeted Therapies
Targeted therapy, or molecular targeted therapy, is a brain cancer treatment that uses drugs to target specific molecules on cancer cells to stop their growth and ability to spread. Some targeted therapies can act as radiosensitizers. A radiosensitizer is an agent or molecule that can enhance the cytotoxic effects of radiation in cancer cells and improve the outcome of radiation therapy. Biomarker testing and tumor profiling can help identify targeted treatment options and clinical trials available to glioblastoma patients.
Immunotherapy
Immunotherapy, such as immune checkpoint inhibitors, cancer vaccines, immune system modulators, T-cell transfer therapy and monoclonal antibodies, is designed to use the body’s immunity against tumor cells.

Support and Resources for Glioblastoma Patients and Caregivers
Many resources are available to help patients and caregivers navigate the challenges of glioblastoma. From transportation and lodging to planning treatment visits, answers to frequently asked questions, and inspiring patient stories, click below for patient support and resources.
Glioblastoma Specialists
The multidisciplinary team at the Ivy Brain Tumor Center at Barrow Neurological Institute spans the entire spectrum of specialty clinicians and investigators. Collectively, we represent the nation’s largest early-phase clinical trials team focusing exclusively on brain tumors, such as glioblastoma. Our team works together seamlessly to accomplish the same goal – to find a cure for glioblastoma through cutting-edge brain cancer research and therapies. Click below to see our team of brain tumor specialists.
Medically Reviewed By: Yoshie Umemura, MD and Matthew Smith-Cohn, DO



