
Newsroom
Ivy Brain Tumor Center and SonALAsense Announce Positive Initial Results of the First-in-Human Phase 0/1 Clinical Trial of Non-Invasive Sonodynamic Therapy for Recurrent Glioblastoma
Trial provides proof of concept for this new metabolically targeted, rapidly acting technology platform for brain tumors and other lethal cancers
PHOENIX, Ariz., Sept. 20, 2021 – The Ivy Brain Tumor Center at Barrow Neurological Institute, the largest early-phase drug development program for brain cancer in the world, and SonALAsense, a pioneer in developing non-invasive sonodynamic therapy (SDT) for managing fatal cancers so patients can become survivors, today announced positive initial results of the first-in-human Phase 0/1 clinical trial of SDT in recurrent glioblastoma patients. The initial results indicate SDT rapidly leads to targeted oxidative stress and cell death in human glioblastoma tissue. SDT was well-tolerated in all patients.
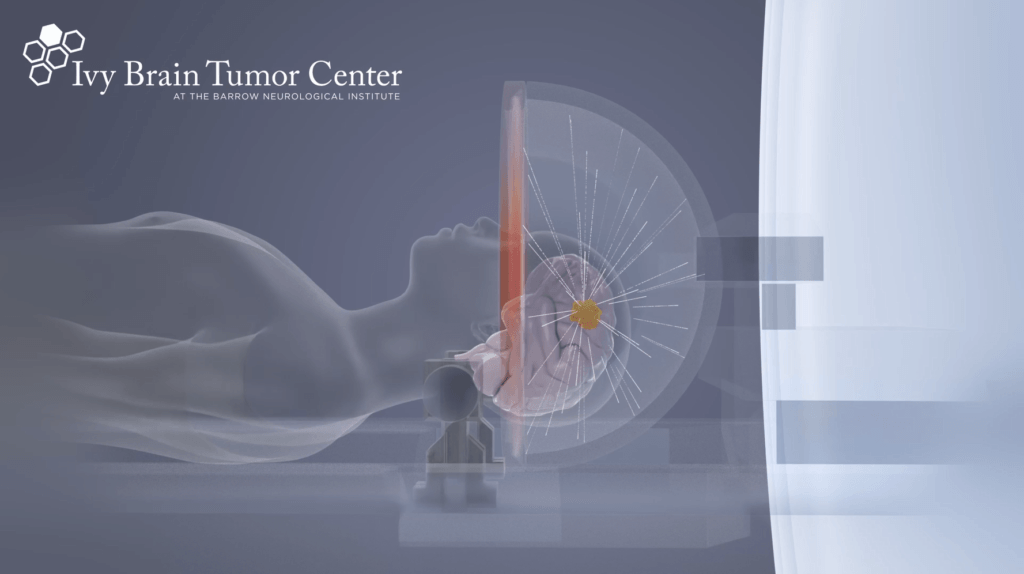
The Ivy Brain Tumor Center dosed its first patient in March 2021 and is currently evaluating SDT using Insightec’s magnetic resonance-guided focused ultrasound (MRgFUS) technology in combination with SonALAsense’s proprietary intravenous formulation of 5-aminolevulinic acid (SONALA-001) to investigate the feasibility, safety, and biological effects of the treatment.
Initial results of this study were presented today at the European Society for Medical Oncology Congress during a mini oral session on CNS tumors by Nader Sanai, MD, director of the Ivy Brain Tumor Center and director of neurosurgical oncology at Barrow Neurological Institute.
“Sonodynamic therapy has tremendous potential to become a new nonsurgical therapeutic modality to treat brain tumors,” said Nader Sanai, MD. “We found that MR-guided focused ultrasound combined with SONALA-001 demonstrated rapid and selective cell death in the patients’ tumors, an encouraging discovery of this new noninvasive treatment option.”
“The Ivy Center’s Phase 0/1 study has shown a promising translation of the preclinical results of SDT in patients with glioblastoma. The same biomarkers that were positive in preclinical studies, and contributed to a significant increase in survival in two animal glioma models, are being seen in the clinical trial patient participants,” said Stuart Marcus, MD, PhD, Founder, CEO and CMO of SonALAsense. “SONALA-001 targets only the glioblastoma tumor cells through selective uptake and conversion to a metabolite which is then activated by focused ultrasound, the SDT process, to kill just the tumor itself, not the surrounding tissue – a truly remarkable finding.”
This study is funded by the Ben and Catherine Ivy Foundation and the individual donors of the Barrow Neurological Foundation. Additional clinical trial information can be found at NCT04559685 or the Ivy Brain Tumor Center’s website.
About Ivy Brain Tumor Center
Ivy Brain Tumor Center at the Barrow Neurological Institute in Phoenix, AZ is a non-profit translational research program that employs a bold, early-phase clinical trials strategy to identify new treatments for aggressive brain tumors, including glioblastoma. The Ivy Center’s Phase 0 clinical trials program is the largest of its kind in the world and enables personalized care in a fraction of the time and cost associated with traditional drug development. Unlike conventional clinical trials focusing on single drugs, its accelerated trials program tests therapeutic combinations matched to individual patients. Learn more at IvyBrainTumorCenter.org. Follow the Ivy Brain Tumor Center on Facebook, Instagram, Twitter and LinkedIn.
About SonALAsense
SonALAsense is a clinical-stage company developing sonodynamic therapy (SDT) as a first-in-class, noninvasive drug-device combination for the treatment of recurrent glioblastoma multiforme, diffuse intrinsic pontine gliomas, and other deadly cancers. SDT utilizes MR-guided focused ultrasound (the device) in combination with aminolevulinic acid (a drug) to selectively target and kill tumor cells. For more information about SonALAsense, visit www.sonalasense.com. Follow SonALAsense on Twitter and LinkedIn.
